
This is the page for the project funded under NSF awards
#2141970 and #2141969: RESEARCH-PGR
Extracellular RNA Produced By Plants:
What, Where, How, Who, and Why?
Overview | Background | Impacts | Team | Publications | Outreach
Project Overview
This project investigates the role of secreted RNA in the immune system of plants. The Innes and Meyers laboratories recently discovered that the leaves of plants accumulate RNA in the spaces between cells and on their surfaces. Although we usually think of RNA as a molecule that can direct cells to synthesize specific proteins (e.g., the mRNA in COVID vaccines directs our cells to make SARS-CoV2 spike protein), some RNAs serve other functions. Analysis of the base sequences of plant extracellular RNAs revealed that these RNAs are diverse in sequence, but do not appear to encode proteins.
The discovery of extracellular non-coding RNA in plants raises two fundamental questions that this project will address: 1) how do plants secrete RNA? and 2) what is the function of this RNA? It takes a large amount of energy for cells to secrete RNA, thus this secreted RNA must benefit the plant in some way. This project will test the hypothesis that secreted RNA functions to protect plants from infection by fungi and bacteria.

GFP fluorescence from GFP-PEN1 EVs using a confocal fluorescence microscope.
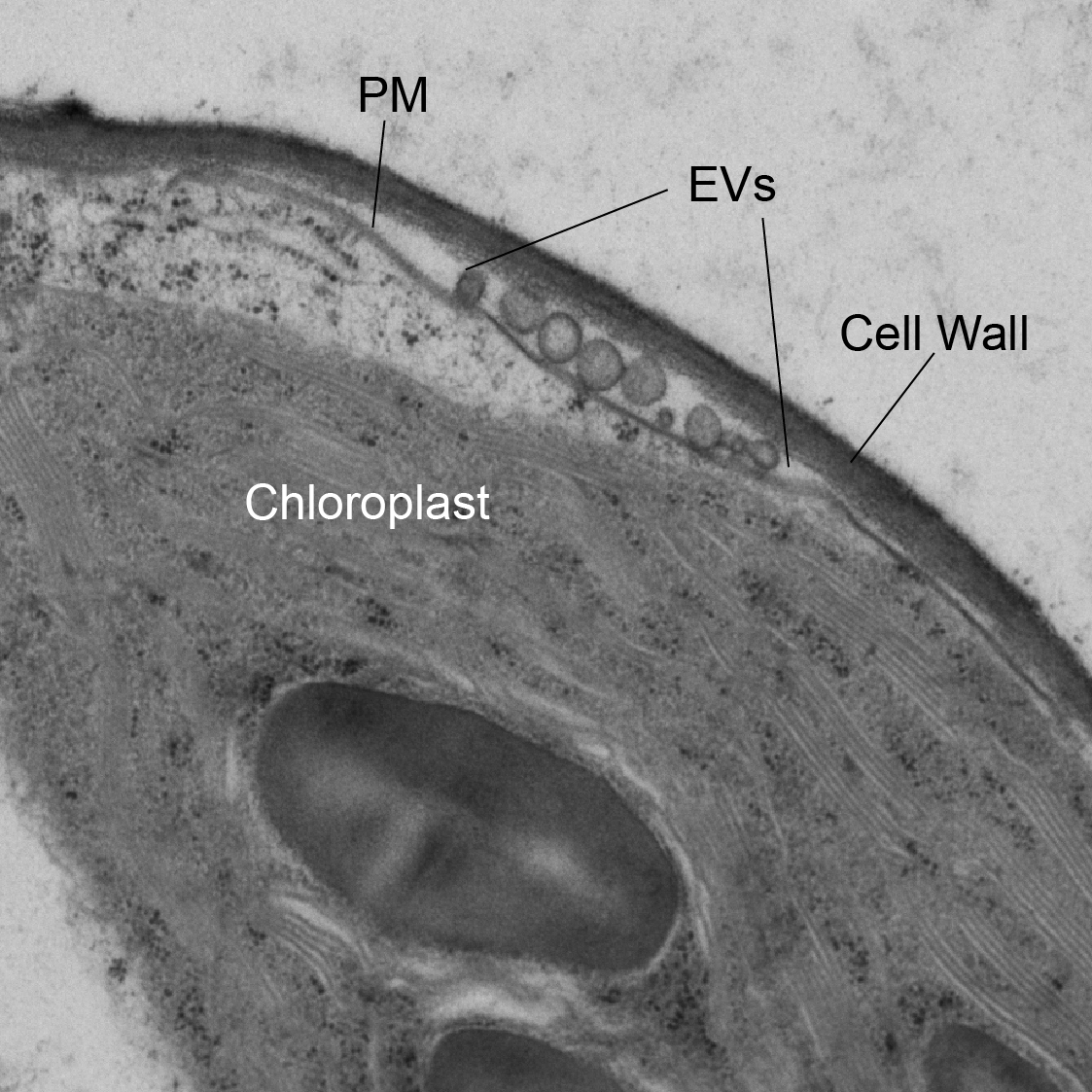
The Innes and Meyers laboratories recently discovered that the apoplast of Arabidopsis leaves contains abundant long non-coding RNAs, including circular RNAs, as well as small RNAs. These RNAs are bound to protein particles, which protects them against degradation. Notably, this extracellular RNA (exRNA) is highly enriched in the post-transcriptional modification N6-methlyadenine (m6A). These discoveries raise fundamental questions about plant biology: Are there specific exRNAs that are broadly conserved across plant species? How are exRNAs secreted, and are post-transcriptional modifications central to this process? And why do plants produce exRNAs? Do they play a fundamental role in plant-microbe interactions?
To answer these questions, exRNA will be purified from the apoplast and leaf surfaces of seven diverse species: Arabidopsis, soybean, tomato, lettuce, pineapple, rice, and maize, which were chosen based on their phylogenetic diversity, genomic resources, importance as crops, and diversity in physiology. These exRNAs will be analyzed using both RNA-seq and sRNA-seq, which will allow identification of RNAs that are conserved between species. To assess whether m6A or other modifications are required for secretion, transgenic plants that express exRNAs that lack modification sites will be tested for their secretion efficiency. To investigate additional requirements for exRNA secretion, the exRNA content in Arabidopsis and rice plants with mutations in known RNA binding proteins and secretory pathway genes will be analyzed. Lastly, to assess whether exRNAs contribute to immunity, mutants compromised in exRNA secretion will be tested for resistance to fungal and bacterial pathogens.

Density-purified Arabidopsis EVs.
Background of the Project
We will isolate and characterize exRNA from the apoplast and from the surface of leaves from diverse crop species, chosen based on their phylogenetic diversity, genomic resources, importance as crops, and diversity in physiology. These exRNAs will be analyzed using both RNAseq and sRNAseq, which will allow us to identify RNAs that are conserved between species. We will also assess how the exRNA content changes in response to pathogen perception, as we hypothesize that exRNAs play an integral role in defense. We will characterize post-transcriptional modifications on exRNA, including m6A, 5-methylcytosine, N-glycan additions, and 3’ end modification (e.g., phosphorylation and methylation). To assess whether m6A or other modifications are required for secretion, we will use mutant plants to express exRNAs that lack modification sites and examine their secretion efficiency. To investigate additional requirements for exRNA secretion, we will assess the effect of mutations in known RNA binding proteins and secretory pathway genes on exRNA content in Arabidopsis and rice. Lastly, to investigate potential roles of exRNA in pathogen defense, we will test whether Arabidopsis and rice circular RNAs can function as sponges for fungal sRNAs, and we will also test whether purified exRNA-protein complexes can confer resistance to infection by fungal and bacterial pathogens.

Apoplastic fluid contains long RNAs
Broader Impacts of Our Project
This project will generate a very large dataset of exRNAs found in seven diverse crop species, which will be an invaluable resource for future research on plant-microbe and plant-insect interactions. It will also provide in depth training and career development for two postdoctoral researchers and multiple undergraduates, all of whom will gain experience in science outreach by developing and implementing a hands-on plant science activity targeting third grade classrooms in Bloomington IN and East St. Louis, IL.

Publications
- Borniego, ML, Singla-Rastogi, M, Baldrich, P, Innes, RW et al. 2025. Diverse plant RNAs coat Arabidopsis leaves and are distinct from apoplastic RNAs. PNAS. doi: 10.1073/pnas.2409090121.
- Singla-Rastogi, M, Borniego, ML, Sampangi-Ramaiah, MH, Innes, RW. 2025. A Stepwise Guide to the Isolation and Analysis of Leaf Surface and Apoplastic RNA Using Arabidopsis Rosettes. J Vis Exp. doi: 10.3791/68406.
- Karimi, HZ, Innes, RW. 2022. Molecular mechanisms underlying host-induced gene silencing. Plant Cell. doi: 10.1093/plcell/koac165. Oxford Adademic The Plant Cell, koac165.
- Karimi, HZ, Baldrich P, Rutter, BD, Borniego, L, Zajt, KK, Meyers, BC, Innes, RW et al. 2022. Arabidopsis apoplastic fluid contains sRNA- and circular RNA–protein complexes that are located outside extracellular vesicles. Plant Cell. doi: 10.1093/plcell/koac0435. Oxford Adademic The Plant Cell, koac0435.
- DeFalco, TA. 2022. In or out? The diversity and location of apoplastic RNAs. Plant Cell. doi: 110.1093/plcell/koac045. Oxford Adademic The Plant Cell, koac045.
- Rutter, BD, Innes, RW. 2020. Growing pains: addressing the pitfalls of plant extracellular vesicle research. New Phytol. doi: 10.1111/nph.16725. PubMed PMID: 32506490.
- Baldrich, P, Rutter, BD, Karimi, HZ, Podicheti, R, Meyers, BC, Innes, RW et al. 2019. Plant Extracellular Vesicles Contain Diverse Small RNA Species and Are Enriched in 10- to 17-Nucleotide “Tiny” RNAs. Plant Cell. doi: 10.1105/tpc.18.00872. PubMed PMID:30705133 PubMed Central PMC6447009.
Outreach

We know there is an urgent need to recruit more students into STEM fields, starting with STEM exposure at a young age. As part of our broader impacts, we proposed working with third graders in the JJK Academy program in East Saint Louis, IL, and a public school in Bloomington, IN. This year we couldn’t connect the two third-grade classes. However, we developed a curriculum and worked separately at JJK Academy.
This curriculum aims to expose students to the scientific method using FastPlants. It includes three 1-hour, beginner lessons for which students need no previous knowledge. Some plant knowledge would help, like plants grow from seeds and need water and light, but these topics are discussed in any case.
The first lesson covers (i) what a scientist is (including drawing a scientist pre-assessment), (ii) an intro to the scientific method and hypotheses, and (iii) planting seeds.
Lesson two includes (i) plant observations and measurements and (ii) a lesson on seed diversity.
Lesson three includes (i) plant observations and measurements, (ii) graphing results, (iii) interpreting results and drawing conclusions, and (iv) a post-assessment of drawing a scientist.
A team of seven scientists at different career stages from the Danforth Center visited JJK second graders for three weeks, from December 2nd – 16th, 2022.








